Intercalation of p-toluenesulfonic acid into Mg-Al layered double hydroxide
DOI:
https://doi.org/10.47566/2022_syv35_1-221101Keywords:
Layered double hydroxide, p-toluenesulphonic acid, IntercalationAbstract
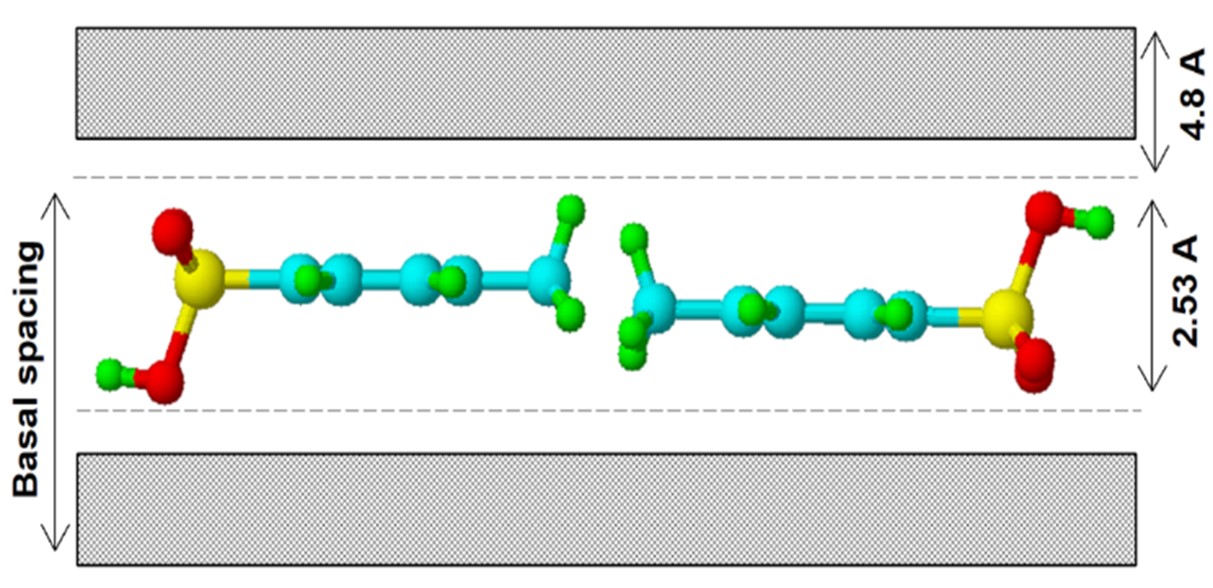
Layered double hydroxide represents a class of materials for selective adsorption and catalysis. In the present work, anionic p-toluenesulfonic acid (PTSA) was successfully incorporated into the interlayer domain of Mg-Al layered double hydroxide (LDH), synthesized by the co-precipitation route. Materials were characterized by X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, Raman spectroscopy, magic angle nuclear magnetic resonance (13C MAS-NMR) spectroscopy and UV-Visible diffuse reflectance (UV-VIS/DR). All samples supported PTSA intercalation at room temperature in the basal space, the interlayer distance increased by the intercalations of the above mentioned according to the arrangement of PTSA molecules in the interlayer. The LDH-PTSA structure collapsed at 400 °C and the organic portion of those materials occurred by partial combustion, which in turn gives rise to reduced species, such as carbon and sulfide anions.
References
.[1]. A. Vaccari, Catal. Today 41, 53 (1998).
https://doi.org/10.1016/S0920-5861(98)00038-8
.[2]. M. Ajat, K. Yusoff, M. Zobir Hussein, Curr. Nanosci. 4, 391 (2008).
https://doi.org/10.2174/157341308786306161
.[3]. D. Tichit, C. Gerardin, R. Durand, B. Coq, Top. Catal. 39, 89 (2006).
https://doi.org/10.1007/s11244-006-0041-6
.[4]. V. Constantino, T. Pinnavaia, Catal. Lett. 23, 361 (1994).
https://doi.org/10.1007/BF00811370
.[5]. M.J. Climent, A. Corma, S. Iborra, K. Epping, A. Velty, J. Catal. 225, 316 (2004).
https://doi.org/10.1016/j.jcat.2004.04.027
.[6]. A. Chen, H. Xu, Y. Yue, W. Hua, W. Shen, Z. Gao, Appl. Catal. A-Gen. 274, 101 (2004).
https://doi.org/10.1016/j.apcata.2004.05.033
.[7]. W. Tongamp, Q. Zhang, F. Saito, J. Mater. Sci. 42, 9210 (2007).
https://doi.org/10.1007/s10853-007-1866-5
.[8]. V.R. Choudhary, D. Dumber, B. Uphade, V. Narkhede, J. Mol. Catal. A Chem. 215, 129 (2004).
https://doi.org/10.1016/j.molcata.2004.01.009
.[9]. L. Ren, J. He, S. Zhang, D.G. Evans, X. Duan, J. Mol. Catal. B Enzym. 18, 3 (2002).
https://doi.org/10.1016/S1381-1177(02)00008-5
.[10]. L. Vieille, M. Moujahid, C. Taviot-Guého, J. Cellier, J.P. Besse, F. Leroux, J. Phys. Chem. Solids 65, 385 (2004).
https://doi.org/10.1016/j.jpcs.2003.08.029
.[11]. W. Yang, Y. Kim, P.K.T. Liu, M. Sahimi, T.T. Tsotsis, Chem. Eng. Sci. 57, 2945 (2002).
https://doi.org/10.1016/S0009-2509(02)00185-9
.[12]. J.T. Kloprogge, D. Wharton, L. Hickey, R.L. Frost, Am. Miner. 87, 623 (2002).
https://doi.org/10.2138/am-2002-5-604
.[13]. V. Rives, Mater. Chem. Phys. 75, 19 (2002).
https://doi.org/10.1016/S0254-0584(02)00024-X
.[14]. R. Trujillano, M.J. Holgado, F. Pigazo, V. Rives, Physica B Condens. Matter 373, 267-273 (2006).
https://doi.org/10.1016/j.physb.2005.11.154
.[15]. H.A. Prescott, Z-J Li, E. Kemnitz, A. Trunschke, J. Deutsch, H. Lieske, A. Auroux, J. Catal. 234, 119 (2005).
https://doi.org/10.1016/j.jcat.2005.06.004
.[16]. A. Corma, V. Fornés, F. Rey, J. Catal. 148, 205 (1994).
https://doi.org/10.1006/jcat.1994.1202
.[17]. G. Fornasari, M. Gazzano, D. Matteuzi, F. Trifiro, A. Vaccari, Appl. Clay Sci. 10, 69 (1995).
https://doi.org/10.1016/0169-1317(95)00022-V
.[18]. Y. Lin, J. Wang, D.G. Evans, D. Li, J. Phys. Chem. Solids 67, 998 (2006).
https://doi.org/10.1016/j.jpcs.2006.01.016
.[19]. M.A. Ulibarri, F.M. Labajos, V. Rives, R. Trujillano, W. Kagunya, W. Jones, Inorg. Chem. 33, 2592 (1992).
https://doi.org/10.1016/j.jpcs.2006.01.016
.[20]. K. Zou, H. Zhang, X. Duan, Chem. Eng. Sci. 62, 2022 (2007).
https://doi.org/10.1016/j.ces.2006.12.041
.[21]. B. Coq, J.M. Planeix, V. Brotons, Appl. Catal. A-Gen. 173, 175 (1998).
https://doi.org/10.1016/S0926-860X(98)00177-X
.[22]. K. Chibwe, W. Jones, Chem. Comm. 1989, 926 (1989). https://doi.org/10.1039/C39890000926
.[23]. Z.P. Xu, R. Xu, H.C. Zeng, Nano lett. 12, 703 (2001). https://doi.org/10.1021/nl010045d
.[24]. X. Hou, D.L. Bish, S.L. Wang C.T. Johnston, R.J. Kikpatrick, Am. Miner. 88, 167 (2003).
https://doi.org/10.2138/am-2003-0120
.[25]. M. S. San Román, M.J. Holgado, C. Jaubertie, V. Rives, Solid State Sci. 10, 1333 (2008).
https://doi.org/10.1016/j.solidstatesciences.2008.01.026
.[26]. M. Ristova, L. Pejov, M. Žugic, B. Soptrajanov, J. Mol. Struct. 482-483, 647 (1999).
https://doi.org/10.1016/S0022-2860(98)00890-4
.[27]. J.M. Alía, H.G.M. Edwards, B.M. Kiernan, Spectrochim. Acta A Mol. Biomol. Spectrosc. 60, 1533 (2004).
https://doi.org/10.1016/j.saa.2003.08.016
.[28]. K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds (Wiley, New York, 1986).
.[29]. M. Moujahid, J. Inacio, J.P. Besse, F. Leroux, Micropor. Mesopor. Mat. 57, 37 (2003).
https://doi.org/10.1016/S1387-1811(02)00505-X
.[30]. D.J. Zalewski, S. Alerasool, P.K. Doolin, Catal. Today 53, 419 (1999).
Downloads
Published
Issue
Section
License
Copyright (c) 2022 Maria Elena Manríquez-Ramírez, Carmen Magdalena Reza-San Germán, Miriam Estrada-Flores

This work is licensed under a Creative Commons Attribution 4.0 International License.
©2025 by the authors; licensee SMCTSM, Mexico. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).





