Spectroscopic study of silver nanoparticles passivated with the conjugated polymer PEDOT:PSS
DOI:
https://doi.org/10.47566/2021_syv34_1-211101Keywords:
Silver nanoparticles, PEDOT:PSS, Optical properties, Electrical conductivityAbstract
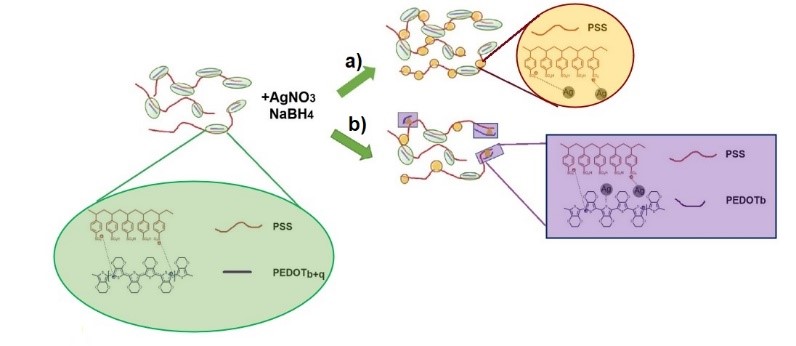
Silver nanoparticles AgNPs were synthesized using sodium boronhydride as reductant and poly(ethylendioxithiophene): poly(sodium estiren sulfonate) PEDOT:PSS, as stabilizer. The following synthetic parameters were varied: 1) PEDOT/PSS relative content, using three different commercial formulations; and 2) molar ratio between the silver salt and the reductant. By UV-Vis spectroscopy, the formation of the AgNPs was corroborated with the plasmonic band at ~ 400 nm, moreover changes in the PEDOT absorption were observed with the richer PSS formulations and the salt/reductant ratio was ~1, which according to Raman can be associated with a conformational change to a more benzenoid structure. In the other conditions, the spectroscopic properties are similar to those of nanoparticles prepared with only PSS. Based on the results, we consider that the AgNPs are stabilized with the PSS that is in excess, out of the conductive PEDOT:PSS domains, when the formulation contains more PSS or when low amounts of NaBH4 are used. Increasing the NaBH4 content, the AgNPs are formed also inside the PEDOT:PSS domains. The optical and electrical properties of the NPs are thus modulated depending on the preferent stabilization mechanism.
References
. S. Kirchmeyer, K. Reuter, J.C. Simpson, Poly(3,4-Ethylene-dixythiophene)-Scientific Importance, Remarkable Properties, and Applications. In: Conjugated Polymers. Theory, Synthesis, Properties and Characterization – Handbook of Conducting Polymers, Eds. T.A. Skotheim, J.R. Reynolds, 3rd ed. (CRC Press, USA, 2007).
https://doi.org/10.1201/9781420043594
. W. Lövenich, Polym. Sci. Ser. C 56, 135 (2014).
https://doi.org/10.1134/S1811238214010068
. G. Huseynova, Y.H. Kim, J.-H. Lee, J. Lee (2020) J. Inf. Display 21, 71 (2020).
https://doi.org/10.1080/15980316.2019.1707311
. K.J. Moreno, I. Moggio, E. Arias, I. Llanera, S.E. Moya, R.F. Ziolo, H. Barrientos, J. Nanosci. Nanotechn. 9, 3987 (2009).
https://doi.org/10.1166/jnn.2009.215
. R.G. Meléndez, K.J. Moreno, I. Moggio, E. Arias, A. Ponce, I. Llanera, S.E. Moya, Mat. Sci. Forum 644, 85 (2010).
https://doi.org/10.4028/www.scientific.net/MSF.644.85
. S. Woo, J.H. Jeong, H.K. Lyu, Y.S. Han, Y. Kim, Nanoscale Res. Lett. 7, 641 (2012).
https://doi.org/10.1186/1556-276X-7-641
. P. Morvillo, A.d.G. del Mauro, G. Nenna, R. Diana, R. Ricciardi, C. Minarini, Energy Procedia 60, 13 (2014).
https://doi.org/10.1016/j.egypro.2014.12.336
. I. Nuramdhani, M. Jose, P. Samyn, P. Adriaensens, B. Malengier, W. Deferme, G. De Mey, L. Van Langenhove, Polymers 11, 345 (2019).
https://doi.org/10.3390/polym11020345
. A. Pyatenko, M. Yamaguchi, M. Suzuki, J. Phys. Chem. C 111, 22, 7910 (2007). https://doi.org/10.1021/jp071080x
. Y. Tan, Y. Li, D. Zhu, Noble Metal Nanoparticles. In: Encyclopedia of Nanoscience and Nanotechnology, Vol. 7, Ed. H. S. Nalwa (Los Angeles, American Scientific Publishers, 2004), pp. 9-40.
https://doi.org/10.1166/000000004323037053
. Z. Fan, D. Du, H. Yao, J. Ouyang, ACS Appl. Mater. Interfaces 9, 11732 (2017).
https://doi.org/10.1021/acsami.6b15158
. M. Marzocchi, I. Gualandi, M. Calienni, I. Zironi, E. Scavetta, G. Castellani, B. Fraboni, ACS Appl. Mater. Interfaces 7, 17993 (2015).
https://doi.org/10.1021/acsami.5b04768
. A. Vázquez-López, A. Yaseen, D. Maestre, J. Ramírez-Castellanos, E.S. Marstein, S. Z. Karazhanov, A. Cremades, Molecules 25, 695 (2020).
https://doi.org/10.3390/molecules25030695
. M.N. Gueyea, A. Carella, J. Faure-Vincent, R. Demadrille, J.-P. Simonato, Prog. Mater. Sci. 108, 100616 (2020).
Downloads
Additional Files
Published
Issue
Section
License
Copyright (c) 2021 The authors; licensee SMCTSM.

This work is licensed under a Creative Commons Attribution 4.0 International License.
©2026 by the authors; licensee SMCTSM, Mexico. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).





